

 Principal |
Mapa do Site |
Adicione aos Favoritos |
Indique o site |
Contato
Principal |
Mapa do Site |
Adicione aos Favoritos |
Indique o site |
Contato

|

 Principal |
Mapa do Site |
Adicione aos Favoritos |
Indique o site |
Contato
Principal |
Mapa do Site |
Adicione aos Favoritos |
Indique o site |
Contato
|
| A Farmabrasilis | O que Fazemos | Pesquisa e Desenvolvimento | Publicações e Monografias | Ensaios Clinicos | Notícias | Contato |
 |

01/10/2008
NATURAL KILLER CELL ACTIVITY, LYMPHOCYTE PROLIFERATION AND CYTOKINE PROFILE IN TUMOR-BEARING MICE TREATED WITH MAPA, A MAGNESIUM AGGREGATED POLYMER FROM Aspergillus oryzae
G. Z. Justo1, N. Durán2 and M. L. S. Queiroz1
1Departamento de Farmacologia and Hemocentro, Faculdade de Ciências Médicas, Universidade Estadual de Campinas (UNICAMP), C.P. 6111, CEP 13083-970, Campinas, SP, Brazil. 2Instituto de Química, Biological Chemistry Laboratory, Universidade Estadual de Campinas (UNICAMP), CEP 13083-970, Campinas, SP, Brazil. e-mail : contato@farmabrasilis.org.br
Immunopharmacology and Immunotoxicology
Volume 25, Number 3 / 2003
Pages :305-319
ABSTRACT
The present study examined the effects of MAPA, an antitumor aggregated polymer of protein magnesium ammonium phospholinoleate-palmitoleate anhydride, isolated from Aspergillus oryzae, on concanavalin A-induced spleen cell proliferation, cytokine production and on NK cell activity in Ehrlich ascites tumor-bearing mice. The EAT growth led to diminished mitogen-induced expansion of spleen cell populations and total NK activity. This was accompanied by striking spleen enlargement, with a marked increase in total cell counts. Moreover, a substantial enhancement in IL-10 levels, paralleled by a significant decrease in IL-2 was observed, while production of IL-4 and IFN-g was not altered. Treatment of mice with 5 mg/kg MAPA for 7 days promoted spleen cell proliferation, IL-2 production and NK cell activity regardless of tumor outgrowth. In addition, MAPA treatment markedly enhanced IFN-g levels and reduced IL-10 production relative to EAT mice. A 35% reduction in splenomegaly with normal number of nucleated cells was also found. Altogether, our results suggest that MAPA directly and/or indirectly modulates immune cell activity, and probably disengages tumor-induced suppression of these responses. Clearly, MAPA has an impact and may delay tumor outgrowth through immunotherapeutic mechanisms.
INTRODUCTION
Progressive tumor growth in humans and animal models is frequently accompanied by a concomitant immunosuppression regardless of tumor location and aetiology (1,2). Additionally, down-regulation of cytotoxic cells, such as T lymphocytes and natural killer (NK) cells, by developing neoplasmas may negatively affect the final outcome of the disease (3-5).
One explanation for the evasion of host defenses by tumors is the production of soluble factors affecting the function of host cells involved in immunity. In this respect, different tumor-derived factors may affect the function of lymphocytes, macrophages and natural killer (NK) cells, or may enhance the expansion of cells with down-regulatory properties (2,4,5).
In addition, the production of factors in abnormal amounts by tumor-bearing hosts may alter normal cytokine network and cause a deleterious imbalance of the immune system (6-9). Previous reports have shown that the growth of the Ehrlich ascites tumor (EAT) results in a dysfunction of T and NK cell immunosurveillance, and that macrophages and natural suppressor cells are in part responsible for this suppressive effect (10-13).
Furthermore, studies suggest that the inactivation of immune responses involving T lymphocytes and NK cells may be mediated partly by a down-regulation of interleukin-2 (IL-2) production (4,14). In this connection, recent studies on this tumor model have found early tumor effects on T lymphocytes, accounting for a diminished interferon-g (IFN-g) expression by T cells after in vitro polyclonal stimulation with mitogens (11,15). Therefore, IL-2 and IFN-g seem to be critical to the functional reactivation of host immune cells against the tumor.
The extracellular purified compound isolated from Aspergillus oryzae, referred to as MAPA, was characterized as an aggregated polymeric form of protein magnesium ammonium phospholinoleate-palmitoleate anhydride (MW=316 kDa), having significant in vivo antitumor and antibacterial activities and no toxicity (16-20).
P-MAPA administered to mice and rats transplanted with plasmacytoma (SP-2/0/Ag14), Walker 256 tumor and spontaneous mammary carcinoma (SP-1) mediated significant antitumor effects that could be ascribed to its ability to increase host defense, without directly affecting the tumor cells (18,21).
Of note, mice cured of Walker 256 by MAPA treatment were largely resistant to rechallenge with Walker 256, suggesting a role for T lymphocytes (19,21).
Subsequent studies from our laboratory have shown that this compound improved the survival of EAT-bearing mice and concurrently reduced tumor growth in the peritoneal cavity (22).
Furthermore, evaluation of hematopoiesis in bone marrow and spleen of normal and EAT-bearing mice revealed that the modulatory effect of MAPA on the myelopoietic response may be partly related to its antitumor activities as a possible mechanism for regulation of granulocyte-macrophage production and expression of functional activities (22).
Similar results were obtained in our laboratory, in mice infected with Listeria monocytogenes and treated with MAPA (20).
The cross-regulation of T lymphocytes as well as the reported studies suggesting the role of soluble factors and cellular interactions to inhibit NK cell activity and lymphocyte functions, led us to evaluate the effects of MAPA on the lymphoproliferative response of spleen cells to concanavalin A (Con A) and on NK cell function in EAT-bearing mice.
In addition, we have investigated cytokine induction in Con A-stimulated spleen cells.
MATERIALS AND METHODS
Animals and Mouse Tumor Model
Male BALB/c mice, 6-8 weeks old, were used for experiments. The Ehrlich ascites tumor (EAT) was used for in vivo evaluation of cytokine profile, mitogen-induced spleen cell proliferation, NK cell activity and changes in spleen weight and cellularity.
Animal experiments were approved by the UNICAMP Institutional Animal Care and Use Committee that follows the recommendations of the Canadian Council on Animal Care (23).
Ehrlich ascites tumor was maintained in BALB/c mice in ascites form by serial transplantation. Tumor cell suspensions were prepared in balanced salt solution at pH 7.4 to final concentrations of 6 x 107 viable cells/ml. In all experimental protocols described, mice were inoculated intraperitoneally (i.p.) on day zero with 6 x 106 viable tumor cells per mouse in a volume of 0.1 ml. Viability, assessed by the trypan blue dye exclusion method, was always found to be 95% or more.
Drug and Treatment Regimen
The biosynthesis of MAPA was carried out from selected cultures of Aspergillus oryzae and purified according to Durán and Nunes (16) and Durán et al. (19). The title compound (P-MAPA) is a white solid obtained as fine microcrystals in the form of an aggregated polymer after 120 h of culturing in appropriated conditions as previously described (16,19). The compound was supplied in balanced salt solution at pH 7.4 and diluted immediately before use in appropriate concentration.
Doses of 5.0 mg/kg were administered for 7 consecutive days to groups of normal and tumor-bearing mice by subcutaneous (s.c.) injections of 0.1 ml per mouse.
Drug injections started 24 h after tumor inoculation. This dose was chosen because optimal dose-dependent antitumor and immunomodulatory effects were previously established using doses of MAPA varying from 0.5 to 5.0 mg/kg (22).
This dose response coincided closely with those for optimal antitumor activities against other solid tumors (18,19,21). Moreover, the dose of 5.0 mg/kg MAPA, given prior or after the EAT inoculation, exhibited maximal therapeutic effect (22).
Lymphoproliferative assay, quantitation of cytokine levels, and changes in spleen weight and cellularity were performed on the first day after the last injection. Each experiment included parallel control groups of normal and tumor-bearing mice treated with an equivalent volume of the diluent.
Preparation of Spleen Cell Suspensions
Suspensions of spleen cells from all mice were prepared by gently pressing aseptically removed spleen through a stainless-steel mesh net.
The cells were suspended in RPMI 1640 culture medium (Sigma Chemical Co., St. Louis, USA) supplemented with 5% foetal calf serum (FCS - Sigma) and washed twice.
Red blood cells were lysed with 0.17 M NH4Cl and the remaining cells were again washed three times and counted. Viability was determined by trypan blue exclusion and consistently exceeded 90%.
Mitogen-Induced Proliferation
Splenocytes were suspended at 1 x 106 cells/ml in RPMI 1640 supplemented with 25 mM HEPES, 25 mM sodium bicarbonate, 2 x 10-5 M 2-mercaptoethanol, 2 mM L-glutamine, 100 mg/ml streptomycin, 80 mg/ml gentamycin (enriched medium) and 5% FCS.
Two hundred and fifty microliters of the cell suspensions were seeded into 96-well microtiter plates (Corning, New York, USA) in the presence of 5 mg/ml Con A (Sigma), and incubated at 37oC, in 5% CO2.
After 72 h incubation, cultures were pulsed with 1mCi [3H]TdR (5 Ci/mmol, Amersham, Little Chalfont, UK) for an additional period of 18 h. The cells were then harvested and [3H]TdR uptake estimated by liquid scintillation counting. Data are expressed as mean cpm [3H]TdR incorporation ± SD of triplicate cultures of each animal, corresponding to 8 mice/group.
Induction of Mouse Cytokine Secretion In Vitro
Splenocytes (1 x 106 cells/ml) were suspended in enriched medium supplemented with 5% FCS and seeded into 24-well culture plates (Corning) in the presence of 5 mg/ml Con A. Cell-free supernatants were collected after 48 h of incubation at 37oC, in 5% CO2, and cytokine levels were detected by ELISA.
Quantitation of Cytokine Levels
Cytokines (IL-2, IL-4, IL-10 and IFN-g) were quantitated by sandwich ELISA using the following monoclonal antibodies (mAbs) purchased from Pharmingen (San Diego, USA).
Purified anti-mouse IL-2 mAb (JES6-1A12 - Rat IgG2a), purified anti-mouse IL-4 mAb (BVD4-1D11 - Rat IgG2b), purified anti-mouse IL-10 mAb (JES5-2A5 - Rat IgG1) and purified anti-mouse IFN-g mAb (R4-6A2 - Rat IgG1). Anti-mouse IL-10 mAb (SXC-1 - Rat IgG1), anti-mouse IL-4 mAb (BVD6-24G2 - Rat IgG1), anti-mouse IL-2 mAb (JES6-5H4 - Rat IgG2b) and anti-mouse IFN-g mAb (XMG1.2 - Rat IgG1) were biotinylated.
Recombinant mouse IL-4 (19231W), recombinant mouse IL-10 (19281V), recombinant mouse IL-2 (19211T) and recombinant mouse IFN-g (19301T) were used as standards.
Cytokine determinations were done according to Pharmingens cytokine ELISA protocol. Briefly, microtiter plates (96-well flat-bottom maxisorp microplate - NUNC, Roskilde, DM) were coated overnight with 2.0 mg/ml anti-cytokine mAbs in a coating buffer of 0.1 M NaHCO3, pH 8.2, at 4oC. A blocking step was performed for 2 h at room temperature (Phosphate buffer saline/10% FCS).
After washing, the recombinant standards and the supernatants were added to the coated plates and incubated overnight at 4oC. The plates were washed and then incubated with the biotinylated anti-cytokine detecting antibodies (2.5 mg/ml) for 45 minutes.
After incubation with avidin-peroxidase (Sigma) for 30 minutes, the substrate consisting of 0.4 mg/ml o-phenylenediamine dihydrochloride (Sigma) and 0.003% H2O2 in citrate buffer, pH 4.35, was added.
Reaction was determined by measure of optical density at 492 nm in a Labsystem Immunoreader (Finland) after stopping the reaction using 1 N H2SO4.
Cytokine titers were expressed as pg per ml, calculated by reference to standard curves constructed with known amounts of recombinant cytokines.
Preparation of Effector Cells for the NK Cell Assay
Spleens from mice were collected and passed through a stainless-steel mesh net to obtain single-cell suspensions. The resultant mononuclear cells were isolated from the cell suspension by Ficoll-Hypaque gradient separation (density, 1.077 g/ml; Pharmacia, Piscataway, USA), washed three times and resuspended in enriched medium supplemented with 10% FCS.
Cell suspensions were placed in 150 mm tissue culture dishes and incubated at 37oC, in 5% CO2, for 90 minutes to remove adherent cells. Non-adherent cells were then harvested by gently pipetting. The cells were washed three times and the concentration adjusted to 5 x 106 cells/ml.
Preparation of Target Cells for the NK Cell Assay
YAC-1, a Moloney virus-induced mouse T-cell lymphoma of A/SN origin, was used as target cell in the 4h 51Cr-release assay. Briefly, 5 x 106 pelleted YAC-1 cells were resuspended to 0.2 ml of FCS and labeled with 100 mCi of sodium chromate (51Cr) (IPEN, Brazil) for 90 minutes at 37oC in a shaking water bath.
After labeling, the cells were washed twice with RPMI 1640 culture medium and resuspended at a concentration of 1 x 105 cells/ml in enriched medium supplemented with 10% FCS.
NK Cell Cytolytic Assay
NK activity of effector cells was measured with a 4 h 51Cr-release assay using YAC-1 target cells. Effector cells and targets were dispensed in triplicates into round-bottom microtiter plate wells (Corning) producing effector to target ratios of 50:1, 25:1 and 12.5:1.
Plates were centrifuged at 800 rpm for 5 minutes and incubated 4 h at 37oC in a humidified CO2 incubator. After the incubation period, the plates were centrifuged again at 1200 rpm for 10 minutes and 0.1 ml of the supernatants were collected for radioactivity counts in a Beckman Biograma Counting System (Beckman 5500 B, Irvine, USA).
Spontaneous release was determined by adding 100 labeled target cells to 0.1 ml of medium in the absence of effector cells and were always less than 10% of the maximum release, which was determined by exposure of labeled target cells to 0.05% Tween-20.
Percentage of cytotoxicity, as measured by specific 51Cr release, was calculated by using the formula: (cpm experimental - cpm spontaneous) / (cpm maximal - cpm spontaneous) x 100.
Statistical Analysis
For statistical analysis of changes in spleen weight and cellularity, proliferation of spleen cells, and NK cell activity, a parametric method, the one-way analysis of variance (ANOVA) followed by the Tukey test, was used to compare data among all groups.
of cytokine levels in all groups was done by Kruskal-Wallis nonparametric ANOVA. In case of significant differences, the Dunns Multiple Comparisons test was used to compare single groups. All P values represent two-sided test of statistical significance, which was assigned when P < 0.05. All statistical analyses were performed using the Statistic 5.1 software (StatSoft, Inc.).
RESULTS
Con A-Induced Proliferation of Spleen Cells
Figure 1 shows that the proliferative response of spleen cells to Con A was significantly reduced in tumor-bearing mice on the eighth day after tumor inoculation, in relation to controls (P < 0.01).
Moreover, these EAT-bearing mice showed a striking spleen enlargement with marked increase in spleen cellularity (P < 0.01) (Table 1).
In contrast to the diminished responsiveness of spleen lymphocytes from EAT mice to the mitogen, treatment with 5.0 mg/kg MAPA for 7 days significantly increased proliferation (P < 0.01) (Figure 1).
In addition, a 35% reduction in splenomegaly with normal numbers of nucleated cells was found in these animals (P < 0.01) (Table 1).
MAPA-treated normal mice demonstrated a slight expansion of mitogen-stimulated spleen cells, relative to controls (P < 0.05) (Figure 1), and no changes were observed in spleen weights and total cell counts (Table 1).
NK Cell Activity
The effects of the treatment of mice with seven doses of 5.0 mg/kg MAPA on NK cell activity are presented in Figure 2. In the tumor-bearing group, NK cell function was significantly reduced when compared to controls (P < 0.01).
However, the degree of NK activity was clearly greater in MAPA-treated EAT-bearing mice, at all effector:target cell ratios (P < 0.05).
In addition, although statistically insignificant, there was certainly an upward trend in absolute NK cell activity in MAPA-treated normal mice (Figure 2).
Cytokine Levels
As shown in Figure 3, the administration of 5.0 mg/kg MAPA for seven consecutive days produced significantly higher IL-2 concentrations in normal mice, when compared with all the other groups studied (P < 0.05).
In tumor-bearing mice, IL-2 levels were significantly reduced by approximately 30% relative to controls, on day eight after tumor inoculation (P < 0.05). However, MAPA promoted spleen cell IL-2 production when given to EAT-bearing mice at 5.0 mg/kg doses (P < 0.05) (Figure 3).
The results of IFN-g production, presented in Figure 4, demonstrated that there is no statistical changes in the concentration of this cytokine in untreated tumor-bearing mice and in normal mice treated with 5.0 mg/kg MAPA, as compared with control animals.
In contrast, higher levels of this cytokine were produced by tumor-bearing animals after MAPA administration, when compared with untreated tumor bearers (P < 0.05).
Moreover, compared with the control group, this concentration represented an increase in the amount of IFN-g produced by 45% (P < 0.05).
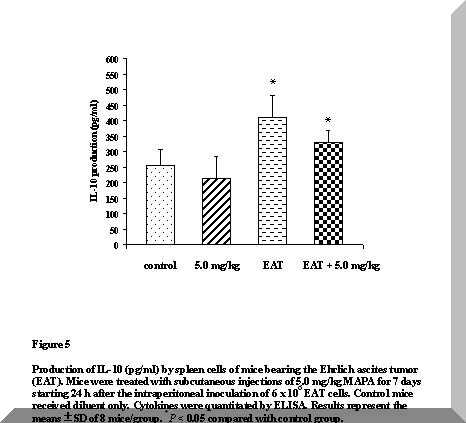
Figure 5 shows a pronounced enhancement in the levels of IL-10 of tumor-bearing animals (P < 0.05). Although this increase was still significant after treatment of these animals with 5.0 mg/kg MAPA, a trend to down-modulate production of this cytokine is suggested.
No differences were detected in IL-10 levels of treated normal mice, when compared with control animals (Figure 5).
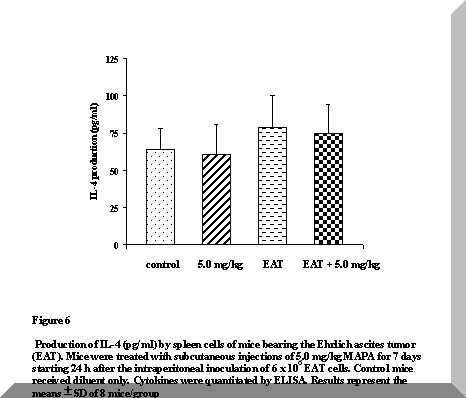
No differences were induced in IL-4 secretion either by the tumor or by the treatment with MAPA (Figure 6). These results are in line with those reported by Segura et al. (11,15), suggesting a progressive loss of T cell functionality in EAT-bearing mice.
DISCUSSION
In recent studies on the immunomodulatory properties of MAPA, our group reported that the dose of 5.0 mg/kg elicited optimal stimulatory effects on bone marrow myelopoiesis, while it markedly inhibited the spleen granulocyte-macrophage colony formation (22).
Moreover, this dose of MAPA, given after tumor inoculation, doubled the life span of EAT-bearing mice and concurrently reduced the intraperitoneal tumor cell burden by 50% (22).
These findings are in line with studies showing that a correlation between the increase in splenic granulocyte-macrophage progenitor cells and the tumor mass exists in this tumor system, and that this phenomenon is closely associated with humoral factors of tumor origin and with the development of suppressor cells (10,12,24).
In this work we extended the study of some additional effects of MAPA administration to EAT-bearing mice on the responsive status of splenic cell populations.
These studies demonstrate that MAPA promotes spleen cell IL-2 production, NK cell activity and Con A-induced lymphocyte proliferation, regardless of tumor outgrowth.
Interesting observations were the significant reduction in spleen weight and the normal number of nucleated cells found in this organ following treatment of tumor-bearing mice with MAPA.
In the Ehrlich tumor model, extramedullar hematopoiesis with splenomegaly has been associated with a strong impairment of lymphoid responses and immunodeficiency (10,12,24). Consistent with earlier reports (4,12), in this experiment the EAT growth led to diminished mitogen-induced expansion of spleen cell populations. This may be accounted by an influx of tumor-induced suppressor macrophages to the spleen, in agreement with the considerable splenomegaly and the reported extramedullar hematopoietic activity in the EAT mouse (4,12,15,22).
On the other hand, there were a significant increase in spleen cell proliferation and a reduction in splenomegaly after MAPA administration.
These results are in keeping with the proposition that MAPA either diminishes the number of, and/or down-regulates the immunosuppressive effects of, tumor-induced macrophages in the spleen.
Natural killer cells represent a small population of lymphocytes exhibiting cytolytic activity against a broad range of tumor cells without prior sensitization (25,26).
NK cells are thought to be key players in tumor rejection in vivo because of their capacity to produce cytokines, particularly IFN-g (27). Relative to IFNs, it is well demonstrated that IFNs or IFN-inducers potentiate NK cell reactivity and activate macrophage functions (28-32).
In addition, several studies reveal that the lymphokine IL-2, alone or in combination with IFNs, also augments the lytic activity of NK cells (29,33-37), which also produce and secrete a variety of immunoregulatory molecules that could synergize with IFNs, IL-2, or IL-2-induced cytokines, for induction of antitumor responses (38,39). IL-2 was first identified as a T cell growth factor (40,41).
In this sense, when spleen cells are stimulated by Con A, binding of the mitogen to the T-cell receptor-CD3 complex activates T lymphocytes giving rise to a cascade of biochemical events that results in cell-cycle entry, expression of IL-2 and IL-2 receptors and finally, cell proliferation (42).
In fact, we observed that normal host IL-2 levels and spleen cell proliferation were increased by MAPA. Likewise, MAPA appears to stimulate IFN-g production and total NK activity.
Conversely, in EAT-bearing mice depressed NK activity and IL-2 secretion were found when IFN-g levels were still normal. These results reinforce literature data suggesting that the decline in NK activity is partly due to a down-regulation of IL-2 production (3,4,13).
In addition, it is clear from our cytokine data that promotion of IL-2 production after MAPA treatment impacts immune cell activity, leading to immune cell proliferation and modulation of their ability to produce cytokines, including IFN-g. This might, in turn, facilitate stimulation of NK cell-mediated cytotoxicity.
At this point, it is worth commenting on the ability of tumor cells to inhibit T cell responses mediated by IFN-g (7-9,11,43-45). In such a context, Segura et al. (11) reported a decrease in the number of Th cells in the spleen of EAT-bearing mice beside a reduction in the number of IFN-g expressing cells. In contrast, no positive cells were found for IL-4 expression (15). Whether MAPA treatment can promote different effects in the various splenic cell populations is worthy of further investigation.
Relative to induction of IL-10 protein in spleen cells, we found that in the EAT-bearing mice, spleen cells stimulated with Con A in vitro produced more IL-10 protein than seen in equivalent cells obtained from control mice. In contrast, we found that in tumor-bearing mice treated with MAPA, IL-10 production was significantly reduced when compared to untreated tumor bearers. IL-10 was first described as an immunoregulatory cytokine, which is produced later after stimulation by T cells or macrophages (46).
However, distinct signalling pathways leading to IL-10 production by various other cells, including B cells, mast cells, and NK cells, have also been shown (47,48). Interestingly, IL-10 was reported to selectively impair the ability of macrophages to provide costimulatory signals for resting T cell proliferation and that this suppression of proliferation is primarily mediated by inhibition of IL-2 production (49).
Even though description of the different splenic cell populations was not assessed in the present work, the data shown here indicate that MAPA directly and/or indirectly induces immune cell proliferation and cytokine production. Clearly, MAPA may function to reverse tumor-induced immunosuppression and may delay tumor outgrowth through immunotherapeutic mechanisms.
ACKNOWLEDGMENTS
The authors are grateful to Mrs. S. C. Oliveira and Mr. R. M. Pereira for their expert technical assistance. This work was supported by grants from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
REFERENCES
1. Chattopadhyay, U., Bhattacharyya, S., and Chakrabarty, N.G., Tumor associated macrophage mediated lysis of autologous tumor cells. Neoplasma, 33: 157, 1986.
2. Nelson, D.S., and Nelson, M., Evasion of host defenses by tumor. Immunol. Cell Biol., 65: 287, 1987.
3. Parhar, R.S., and Lala, P.K., Changes in the host natural killer cell population in mice during tumor development. 1. Kinetics and in vivo significance. Cell. Immunol., 93: 250, 1985.
4. Parhar, R.S., and Lala, P.K., Prostaglandin E2-mediated inactivation of various killer lineage cells by tumor-bearing host macrophages. J. Leukocyte Biol., 44: 185, 1988.
5. Lopez, D.M., Lopez-Cepero, M., Watson, G.A., Ganju, A., Sotomayor, E.M., and Fu, Y-X., Modulation of the immune system by mammary tumor-derived factors. Cancer Invest., 9: 643, 1991.
6. Arteaga, C.L., Hurd, S.D., Winnier, A.R., Johnson, M.D., Fendly, B.M., and Forbes, J.T., Anti-transforming growth factor (TGF)-b antibodies inhibit breast cancer cell tumorigenicity and increase mouse spleen natural killer cell activity. Implications for a possible role of tumor cell/host TGF-b interactions in human breast cancer progression. J. Clin. Invest., 92: 2569, 1993.
7. Gosh, P., Komschlies, K.L., Cippitelli, M., Longo, D.L., Subleski, J., Ye, J., Sica, A., Young, H.A., Wiltrout, R.H., and Ochoa, A.C., Loss of T-helper 1 populations in spleen of mice during progressive tumor growth. J. Natl. Cancer Inst., 87: 1478, 1995.
8. Handel-Fernandez, M.E., Cheng, X., Herbert, L.M., and Lopez, D.M., Down-regulation of IL-12, not a shift from a T helper-1 to a T helper-2 phenotype, is responsible for impaired IFN-g production in mammary tumor-bearing mice. J. Immunol., 158: 280, 1997.
9. Huang, M., Sharma, S., Mao, J.T., and Dubinett, S.M., Non-small cell lung cancer-derived soluble mediators and prostaglandin E2 enhanced peripheral blood lymphocyte IL-10 transcription and protein production. J. Immunol., 157: 5512, 1995.
10. Ruiz de Morales, J., Vélez, D., and Subiza, J.L., Ehrlich tumor stimulates extramedullar hematopoiesis in mice without secreting identifiable colony-stimulating factors and without engagement of host T cells. Exp. Hematol., 27: 1757, 1999.
11. Segura, J.A., Barbero, L.G., and Márquez, J., Early tumor effect on splenic Th lymphocytes in mice. FEBS Lett., 414: 1, 1997.
12. Subiza, J.L., Viñuela, J.E., Rodriguez, R., Gil, J., Figueredo, M.A., and De la Concha, E.G., Development of splenic natural suppressor (NS) cells in Ehrlich tumor-bearing mice. Int. J. Cancer, 44: 307, 1989.
13. Parhar, R.S., and Lala, P.K., Changes in the host natural killer cell population in mice during tumor development. 2. The mechanism of suppression of NK activity. Cell. Immunol., 93: 265, 1985.
14. Chouaib, S., Welte, W., Martelsmann, R., and Dupont, B., Prostaglandin E2 acts at two distinct pathways of T lymphocyte activation: inhibition of interleukin-2 production and down regulation of transferrin receptor expression. J. Immunol., 135: 1172, 1985.
15. Segura, J.A., Barbero, L.G., and Márquez, J., Ehrlich ascites tumor unbalances splenic cell populations and reduces responsiveness of T cells to Staphylococcus aureus enterotoxin B stimulation. Immunol. Lett., 74: 111, 2000.
16. Durán, N., and Nunes, O.D.S., Characterization of an aggregated polymer from Penicillium sp. (PB-73 strain). Brazil. J. Med. Biol. Res., 23: 1289, 1990.
17. Durán, N., Haun, M., Pereira-da-Silva, L., Pisani, R., Pisani, F.J.C., Souza-Brito, A.R.M., Mazetto, M.N., and Nunes, O.D.S., Comparison of the antiviral activity and toxicity of the protein magnesium ammonium phospholinoleate anhydride polymer with other antiviral drugs. Brazil. J. Med. Biol. Res., 23: 1303, 1990.
18. Durán, N., Souza-Brito, A.R.M., Haun, M., De Oliveira, J.A., Hetem, S., Vargas, L., Saavedra, I., and Justo, G.Z., SB-73-immunostimulant. Drugs Fut., 18: 327, 1993.
19. Durán, N., Justo, G.Z., Souza-Brito, A.R.M., Rettori, O., and Vieira-Matos, A.N., SB-73/MAPA: Protein magnesium ammonium phospholinoleate-palmitoleate anhydride. Drugs Fut., 22: 454, 1997.
20. Melo, A., Justo, G.Z., and Queiroz, M.L.S., Stimulation of myelopoiesis in Listeria monocytogenes-infected mice by an aggregated polymer isolated from Aspergillus oryzae. Human Exp. Toxicol., 20: 38, 2001.
21. Durán, N., Justo, G.Z., Queiroz, M.L.S., Vieira-Matos, A.N., and Rettori, O., New perspectives in immunomodulatory therapy of tumor induced by an extracellular aggregated polymer isolated from Aspergillus oryzae. Int. J. Mol. Med., 4: S49, 1999.
22. Justo, G.Z., Durán, N., and Queiroz, M.L.S., Myelopoietic response in tumor-bearing mice by an aggregated polymer isolated from Aspergillus oryzae. Eur. J. Pharmacol., 388: 219, 2000.
23. Olfert, E.D., Cross, B.M., and McWilliam, A.A., In Guide to the care and use of experimental animals, Canadian Council on Animal Care, Vol. 1., p. 1, Ottawa, 1993.
24. Viñuela, J.E., Rodríguez, R., Gil, J., Coll, J., De la Concha, E.G., and Subiza, J.L., Antigen shedding vs. development of natural suppressor cells as mechanism of tumor escape in mice bearing Ehrlich tumor. Int. J. Cancer, 47: 86, 1991.
25. Kim, S., Iizuka, K., Aguila, H.L., Weissman, I.L., and Yokoyama, W.M., In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc. Natl. Acad. Sci. USA, 97: 2731, 2000.
26. Trinchieri, G., Biology of natural killer cells. Adv. Immunol., 47: 187, 1989.
27. Scott, P., and Trinchieri, G., The role of natural killer cells in host-parasite interactions. Curr. Opin. Immunol., 7: 34, 1995.
28. Djeu, J.Y., Heinbaugh, J.A., Holden , H.T., and Herberman, R.B., Augmentation of mouse natural killer cell activity by interferon and interferon inducers. J. Immunol., 122: 175, 1979.
29. Kuribayashi, K., Gillis, S., Kern, D.E., and Henney, C.S., Murine NK cell cultures: effects of interleukin-2 and interferon on cell growth and cytotoxic reactivity. J. Immunol., 126: 2321, 1981.
30. Malkovsky, M., Loveland, M., North, M., Asherson, G., Gao, L., Ward, P., and Fiers, W., Recombinant interleukin-2 directly augments the cytotoxicity of human monocytes. Nature, 325: 262, 1987.
31. Pace, J.L., Russel, S.W., Torres, B.A., Johnson, H.M., and Gray, P.W., Recombinant mouse g interferon induces the priming step in macrophage activation for tumor cell killing. J. Immunol., 130: 2011, 1983.
32. Yamamoto, N., Zou, J.P., Li, X.F., Takenaka, H., Noda, S., Fujii, T., Ono, S., Kobayashi, Y., Mukaida, N., Matsushima, K., Fujiwara, H., and Hamaoka, T., Regulatory mechanisms for production of IFN-g and TNF by antitumor T-cells or macrophages in the tumor-bearing state. J. Immunol., 154: 2281, 1995.
33. Hefeneider, S.H., Conlon, P.J., Henney, C.S., and Gillis, S., In vivo interleukin 2 administration augments the generation of alloreactive cytolytic T lymphocytes and resident natural killer cells. J. Immunol., 130: 222, 1983.
34. Henney, C.S., Kuribayashi, K., Kern, D.E., and Gillis, S., Interleukin-2 augments natural killer cell activity. Nature, 291: 335, 1981.
35. Misawa, E., Sakurai, T., Yamada, M., Hayasawa, H., and Motoyoshi, K., Effects of macrophage colony-stimulating factor and interleukin-2 administration on NK1.1+ cells in mice. Int. J. Immunopharmacol., 22: 967, 2000.
36. Sayers, T.J., and Wiltrout, R.H., Differing roles for interleukin 2 and interferon g in the augmentation of mouse peritoneal natural killer cell activity in vivo. In Natural killer cells and host defenses, edited by E. W. Ades and C. Lopez, p. 88, S. Karger AG, Basel, 1989.
37. Young, H.A., and Ortaldo, J.R., One-signal requirement for interferon-g production by human large granular lymphocytes. J. Immunol., 139: 724, 1987.
38. Allavena, P., Scala, G., Djeu, J.Y., Procopio, A.D., Oppenheim, J.S., Herberman, R.B., and Ortaldo, J.R., Production of multiple cytokines by clones of human large granular lymphocytes. Cancer Immunol. Immunother., 19: 121, 1985.
39. Svedersky, L.P., Nedwin, G.E., Goeddel, D.V., and Palladino Jr, M.A., Interferon-g enhances induction of lymphotoxin in recombinant interleukin 2-stimulated peripheral blood mononuclear cells. J. Immunol., 134: 1604, 1985.
40. Ehrhardt, R.O., Ludviksson, B.R., Gray, B., Neurath, M., and Strober, W., Induction and prevention of chronic inflammation in IL-2-deficient mice. J. Immunol., 158: 566, 1997.
41. Taniguchi, T., Matsui, H., Fujita, T., Takaoka, C., Kashima, N., Yoshimoto, R., and Hamuro, J., Structure and expression of cloned cDNA for human interleukin-2. Nature, 302: 305, 1983.
42. Licastro, F., Davis, L.J., and Morini, M.C., Lectins and superantigens: membrane interactions of these compounds with T lymphocytes affect immune responses. Int. J. Biochem., 25: 845, 1993.
43. Elexpuru, A., Martin-Nieto, J., Jimenez, A., Gomez, C., and Villalobo, A., Ehrlich ascites tumor cells produce a transforming growth factor-beta (TGFb)-like activity but lack receptors with TGFb-binding capacity. Mol. Cell. Biochem., 170: 153, 1997.
44. Stefanski, H.E., and Mathur, A., Decreased expression and function of Vb6+ and Vb14+ T cells is associated with decreased Th1 cytokine production in mice with plasma cell tumors. Tumori, 82: 22, 1996.
45. Tada, T., Ohzeki, S., Utsumi, K., Takiuchi, H., Muramatsu, M., Li, X-F., Shimizu, J., Fujiwara, H., and Hamaoka, T., Transforming growth factor-b-induced inhibition of T cell function. Susceptibility difference in T cells of various phenotypes and functions and its relevance to immunosuppression in the tumor-bearing state. J. Immunol., 146: 1077, 1991.
46. Yssel, H., De Wall Malefyt, R., Roncarolo, M., Abrams, J.S., Lahesmaa, R., Spits, H., and De Vries, J.E., IL-10 is produced by subsets of human CD4+ T cell clones and peripheral blood T cells. J. Immunol., 149: 2378, 1992.
47. Howard, M., and OGarra, A., Biological properties of interleukin 10. Immunol. Today,13: 198, 1992.
48. Mehrotra, P.T., Donnelly, R.P., Wong, S., Kanegane, H., Geremew, A., Mostowski, H.S., Furuke, K., Siegel, J.P., and Bloom, E.T., Production of IL-10 by human natural killer cells stimulated with IL-2 and/or IL-12. J. Immunol., 160: 2637, 1998.
49. Ding, L., and Shevach, E.M., IL-10 inhibits mitogen-induced T cell proliferation by selectively inhibiting macrophage costimulatory function. J. Immunol., 148: 3133, 1992.
Table 1.Effect of 5.0 mg/kg MAPA on Spleen Weight and Total Spleen Cell Count in Mice Bearing the Ehrlich Ascites Tumor (EAT)a
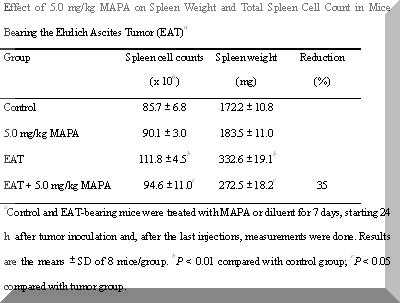
aControl and EAT-bearing mice were treated with MAPA or diluent for 7 days, starting 24 h after tumor inoculation and, after the last injections, measurements were done. Results are the means ± SD of 8 mice/group. bP < 0.01 compared with control group; cP < 0.05 compared with tumor group.
Figure 1. Con A-stimulated splenic lymphocyte proliferation (1 x 106 cells/ml) in the control and Ehrlich ascites tumor-bearing mice (EAT) after treatment with 5.0 mg/kg MAPA for 7 days. Control mice received diluent only. The [3H]TdR uptake was estimated by liquid scintillation counting. Data are expressed as mean cpm [3H]TdR incorporation ± SD of triplicate cultures of each mouse, corresponding to 8 mice/group. Proliferative responses in the absence of Con A (5 mg/ml) were less than 1000 cpm. +P < 0.05 compared with control group; *P < 0.01 compared with control group; #P < 0.01 compared with tumor group.
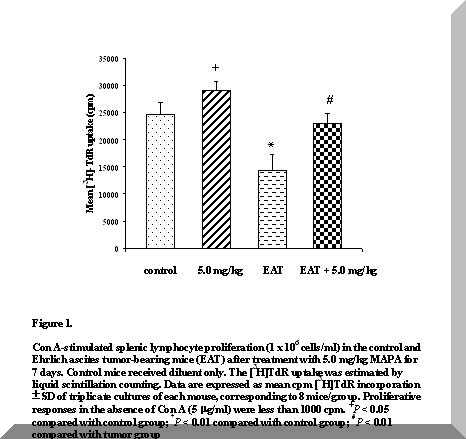
Figure 2. NK cell activity in mice bearing the Ehrlich ascites tumor (EAT). Mice were treated with subcutaneous injections of 5.0 mg/kg MAPA for 7 days starting 24 h after the intraperitoneal inoculation of 6 x 106 EAT cells. Control mice received diluent only. Results represent the means ± SD of 8 mice/group. *P < 0.01 compared with control group; **P < 0.01 compared with tumor group.
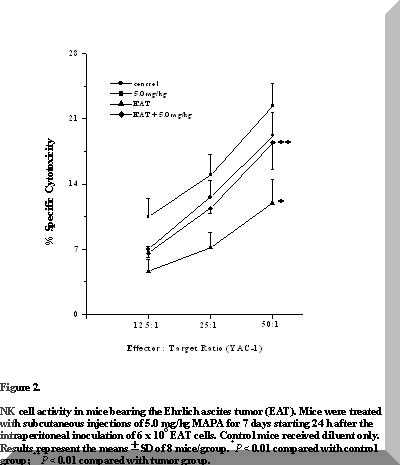
Figure 3. Production of IL-2 (pg/ml) by spleen cells of mice bearing the Ehrlich ascites tumor (EAT). Mice were treated with subcutaneous injections of 5.0 mg/kg MAPA for 7 days starting 24 h after the intraperitoneal inoculation of 6 x 106 EAT cells. Control mice received diluent only. Cytokines were quantitated by ELISA. Results represent the means ± SD of 8 mice/group. *P < 0.05 compared with control group; #P < 0.05 compared with tumor group
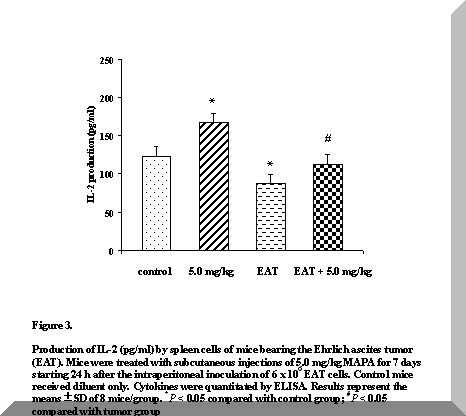
Figure 4. Production of IFN-g (pg/ml) by spleen cells of mice bearing the Ehrlich ascites tumor (EAT). Mice were treated with subcutaneous injections of 5.0 mg/kg MAPA for 7 days starting 24 h after the intraperitoneal inoculation of 6 x 106 EAT cells. Control mice received diluent only. Cytokines were quantitated by ELISA. Results represent the means ± SD of 8 mice/group. *P < 0.05 compared with control and tumor groups.
Figure 5. Production of IL-10 (pg/ml) by spleen cells of mice bearing the Ehrlich ascites tumor (EAT). Mice were treated with subcutaneous injections of 5.0 mg/kg MAPA for 7 days starting 24 h after the intraperitoneal inoculation of 6 x 106 EAT cells. Control mice received diluent only. Cytokines were quantitated by ELISA. Results represent the means ± SD of 8 mice/group. *P < 0.05 compared with control group.
Figure 6. Production of IL-4 (pg/ml) by spleen cells of mice bearing the Ehrlich ascites tumor (EAT). Mice were treated with subcutaneous injections of 5.0 mg/kg MAPA for 7 days starting 24 h after the intraperitoneal inoculation of 6 x 106 EAT cells. Control mice received diluent only. Cytokines were quantitated by ELISA. Results represent the means ± SD of 8 mice/group. 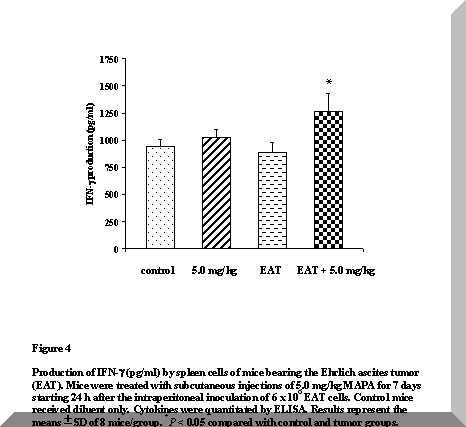


Política de Privacidade | Termo de Uso |
copyright @2006 - farmabrasilis - todos os direitos reservados
|