

 Home |
Site Map |
Add to Favorites Link |
Send page to a Friend |
Contact us
Home |
Site Map |
Add to Favorites Link |
Send page to a Friend |
Contact us

|

 Home |
Site Map |
Add to Favorites Link |
Send page to a Friend |
Contact us
Home |
Site Map |
Add to Favorites Link |
Send page to a Friend |
Contact us
|
| About Us | Products | Research and Development | Publications and Press | Clinical Trials | Latest News | Contact us |
 |

10/07/2008
• 12week multiple-dose toxicity of P-MAPA in rats
Experimental Protocol
• Groups of 10 male (4 weeks old, 100 g BW) and 10 female (4 weeks old, 90-100 g /BW) rats were injected sc in the nape with 1 (LD) or 10 (MD) or 100 (HD) mg/kg of a suspension of P-MAPA into 10 ml/kg for 90 consecutive days. (Figure 1)
Figure 1
All rats were observed twice daily for physiological and behavioral response and for mortality. Body weights (BW) were recorded prior to randomization at the initiation of dosing and daily thereafter. The individual body weight was used to adjust the P-MAPA dose. Food and excreta (faeces and urine) were weighed every day. The water consumption was also evaluated.
• Blood samples from all the animals were analyzed for hematological and serum clinical chemistry evaluations and urine samples were collected r for biochemical analysis, as noted under results.
• Hematology samples were evaluated at laboratory, by use of an automated counter for white blood cells (WBC), red blood cell (RBC) count, hemoglobin (HGB) and hematocrit (HCT).
• A WBC differential analysis was performed for lymphocytes, monocytes, eosinophils, and segmented neutrophils.
• Coagulation time (Ct) also was determined.
The animals were necropsied and the pieces included gross examination of the external surface, all orifices, external surface of organs, and the cranial, thoracic, abdominal, and pelvic cavities were carried out.
Weight of kidneys with adrenal gland, liver, heart, spleen, thymus, brain and reproductive organs of all animals were determined and expressed as body weight ratios.
The following tissues from all groups were preserved in 10% neutral buffered formalin for histopathological evaluation: heart, lungs, trachea, liver, spleen, kidneys, adrenal, urinary bladder, salivary gland, thymus, pituitary, gonads, thyroid/parathyroids, brain, eyes, prostate, seminal vesicles, epididymus, uterus, esophagus, stomach, duodenum, jejunum. ileum, with lymphonodes, cecum, rectum, colon, pancreas, stenebrae, femur, tooth, tongue, and pieces of tissue along skin from the nape where P-MAPA was injected.
Subsequently, these tissues were trimmed, processed embedded in paraffin and sectioned, and slides (5 to 6 um sections) were prepared and stained, for microscopic examination with hematoxylin and aeosin, or other specific stains.
All prepared slides were examined by a veterinary pathologist. Lesions were graded according to severity with a scale of 1?4 (minimal, mild, moderate, or marked).
Data were analyzed by individual animals and the descriptive statistics summarized by groups.
Results
Body and Organ Weight
Initial and final body weights, weight gain, and absolute organ weights were recorded and analyzed.
The analysis of body weight versus time indicates that the growth curves for all groups were not equivalent, but when expressed as weight gain (%) for the study period, the results are different.
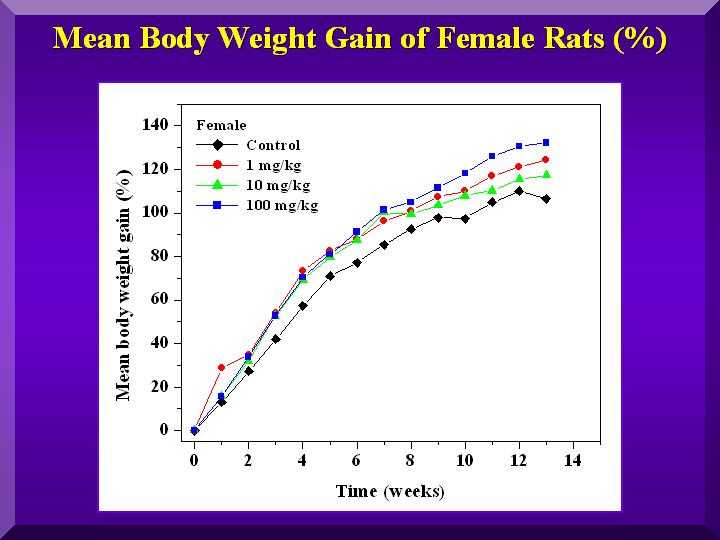
P-MAPA affected the rate of body weight gain in both sexes. Females gained more weight than the controls and the witnesses, in all doses. (Figure 2).
Figure 2
On the other hand, the males gained less weight than the controls and the witnesses only in the high dose (Figure 3).
Figure 3
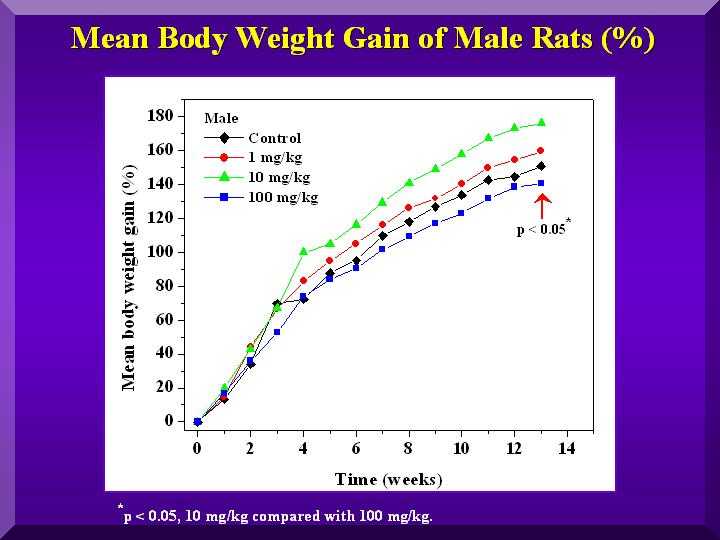
As can be seen from the results 100 mg/kg BW/day (HD) had a greater effect than 10 (MD) and 1 (LD) mg/kg BW/day in both sexes.
Terminal body weight and organs to body (relative) weight ratios computed on the weight after necropsy showed that there were statistical differences in the initial mean body weight among the various groups of male or females animals, mainly in the last group.
The relative weights (weight per 100 g BW) of thymus and spleen of females and males were increased in a dose-dependent way as well as the reproductive organs of males ( Figure 4 and Figure 5).
Figure 4
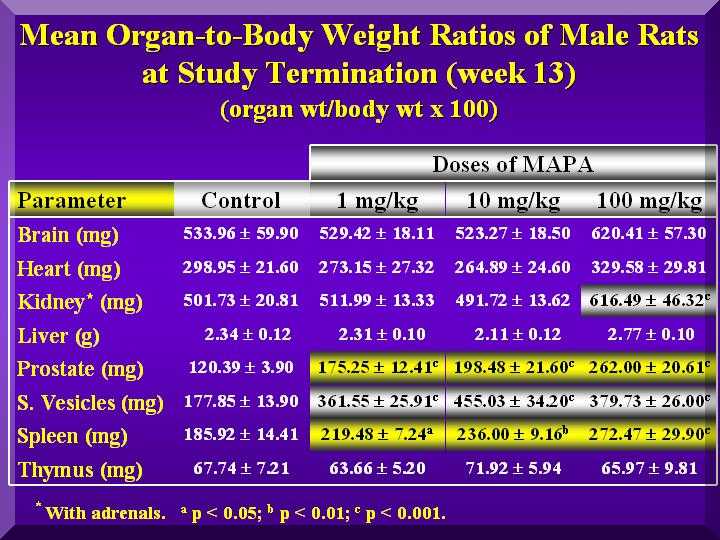
Figure 5
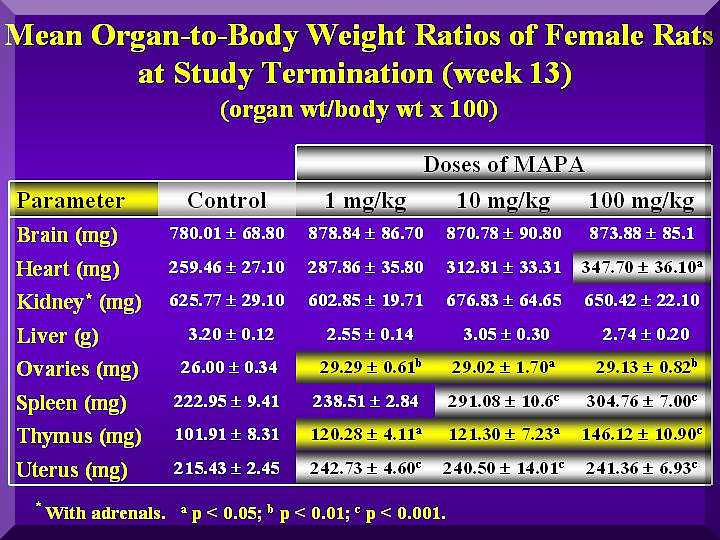
Nevertheless, the increase observed in relative thymus weight in male and female was not statistically significant.
The relative uterus/ovarius and prostata/seminal vesicle weight increased but the increase was not dependent on P-MAPA dose.
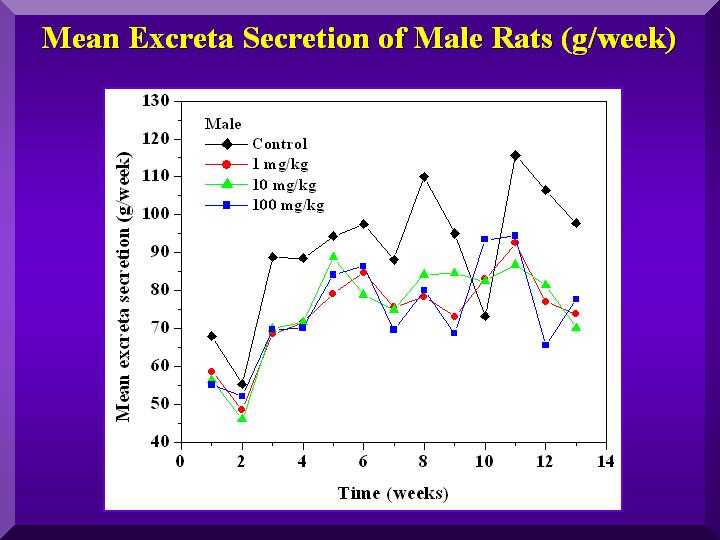
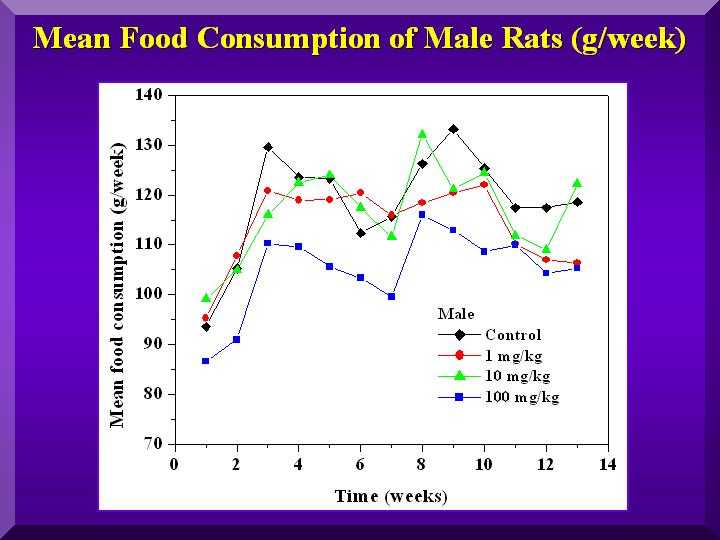
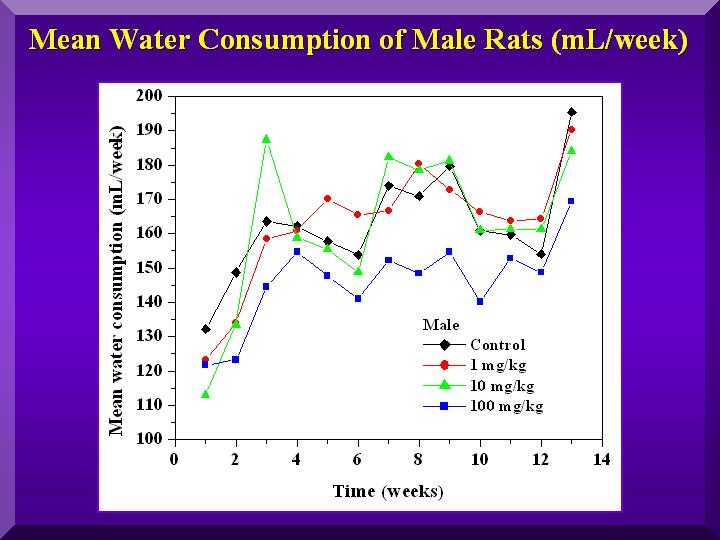
Food and Water Consumption and Excretion
No statistically significant differences were found for all of these parameters.
Tables and graphs
Excretion - males
Mean food consumption - males
Mean water consumption - males
Excretion - females
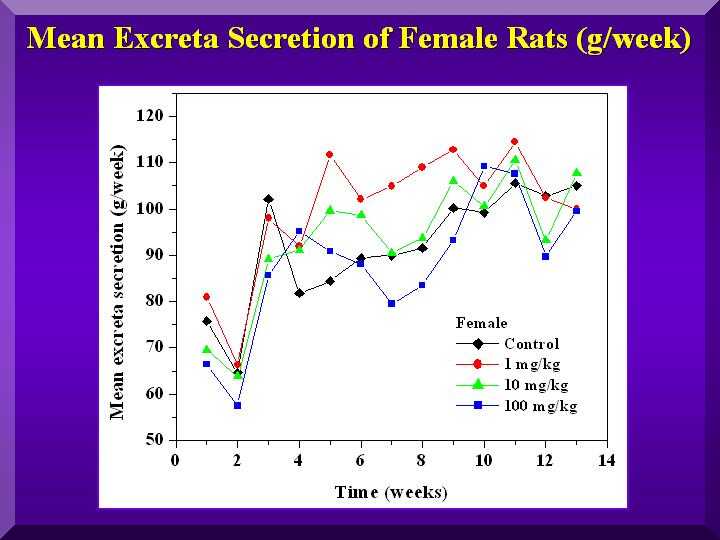
Mean food consumption -females
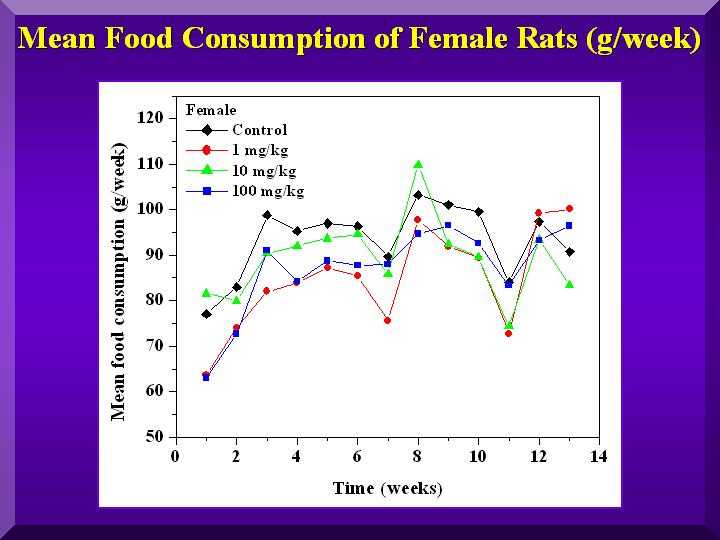
Mean water consumption-females
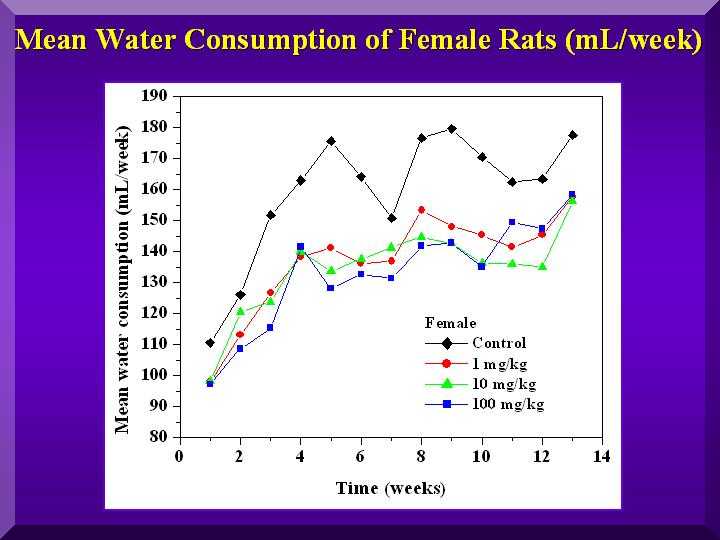
Hematological parameters
In the analysis of the hematological parameters in the P-MAPA treated males and females, the white blood cells were increased in a dose-dependent way.
Hematological parameters - males
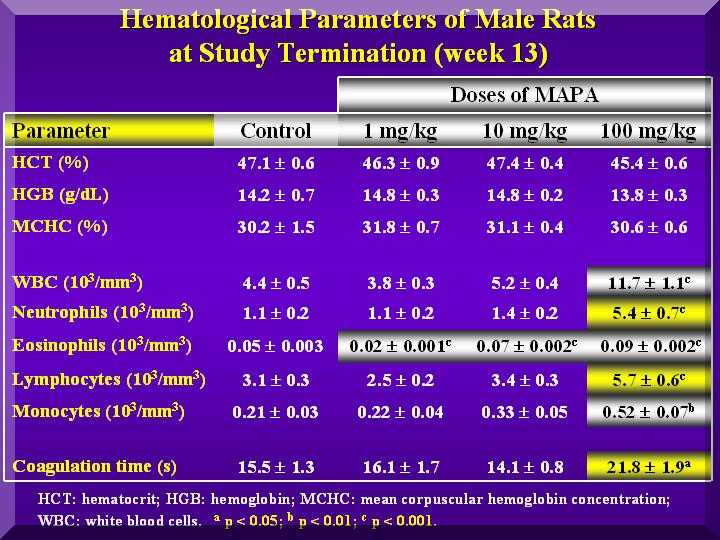
Hematological parameters - females
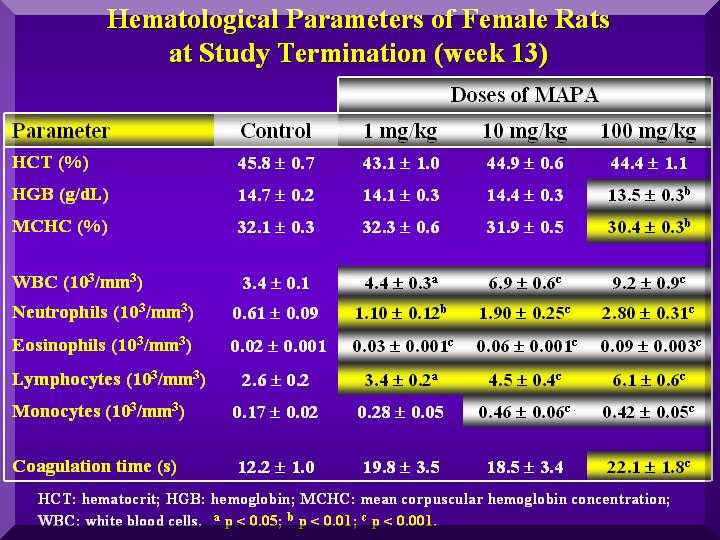
The WBC differential analysis performed showed that the lymphocytes, neutrophils and eosinophils increased with increase of the P-MAPA dose in males and females.
No change in hermatocrit, hemoglobin and red blood cells, were observed except for some cases of HD, in both female and male rats.
Coagulation time was changed in the HD of P-MAPA treated animals.
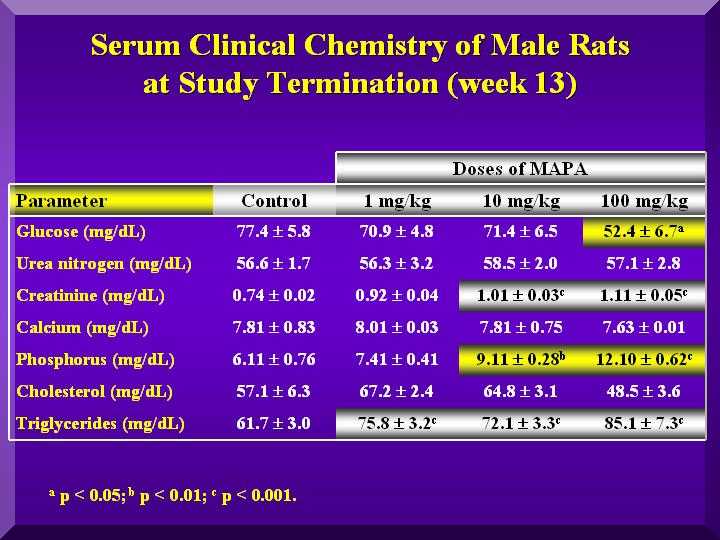
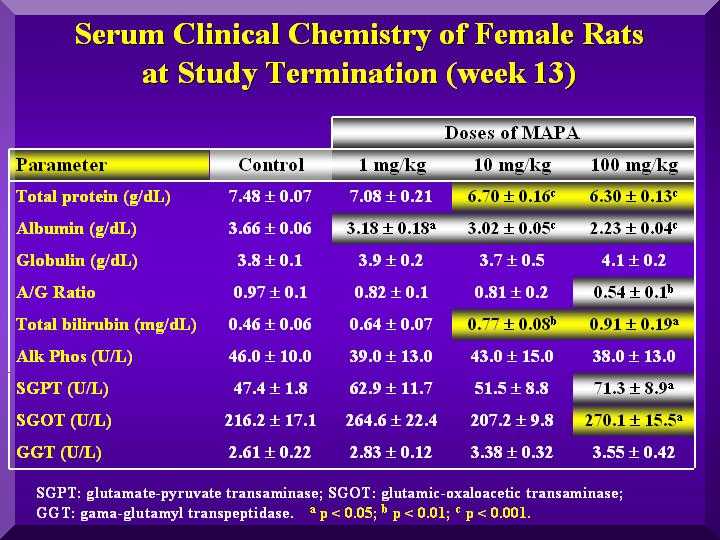
Biochemical Parameters
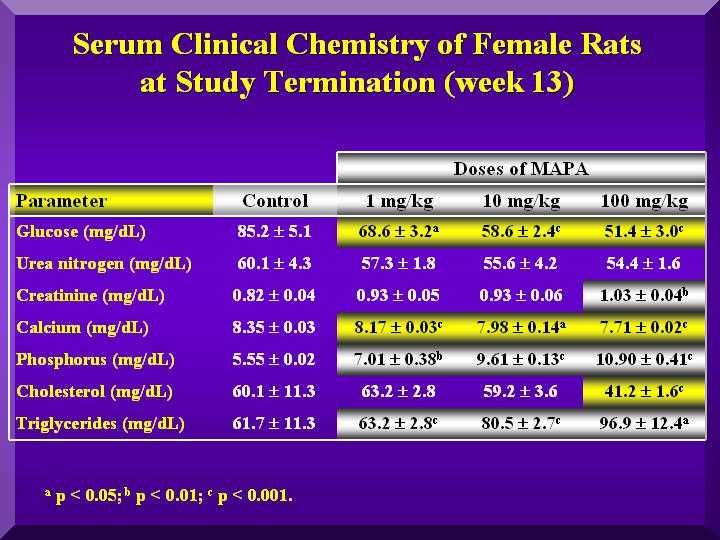
The serum clinical chemistry analysis revealed statistically significant differences.
A dose dependent decrease of total proteins in all animals was observed, but only for females, this decrease was statistically significant.
Globulins increase led to an albumin decrease. Plasmatic calcium levels decreased while inorganic phosphorous levels increased, indicating possible changes in bone metabolism. This alteration however, was not confirmed by histopathological analysis of tooth and femur.
Some change in SGOT, SGPT, Gama-GT and bilirrubin levels were observed, but no P-MAPA dose-dependent was observed.
However, the liver anatomopathological analyses of all the treated animals with P-MAPA did not show any hepatic lesions.
No changes in the cholesterol and triglycerides, urea nitrogen and creatinine levels were observed, indicating no changes on lipids and renal metabolism.
The decrease of glucose levels can not be attributed to the P-MAPA effect, since the determination was done 10 h after sampling. Thus, there was a consumption of substrate.
Tables and graphs
-rats serum biochemical analysis 1 (males)
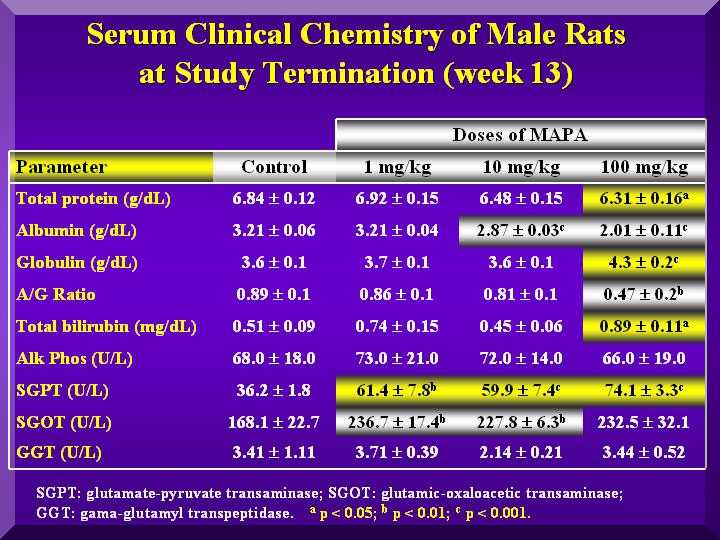
- rats serum biochemical analysis 2 (males
-rats serum biochemical analysis 1 (females)
- rats serum biochemical analysis 2 (females
Anatomopathology
Microscopic evaluation of the tissues from control and treated animals were carried out.
Results
Histological examination of all structures and organs removed from the animals gave normal results. Alterations related to the P-MAPA mode of action will be discussed separately, aimed at the possible organ targets for P-MAPA, and they are the following:
• Morphological diagnosis of the gross intestine revealed that there were no pathological alterations, although an augmented activity of lymphoid tissue associated to the intestine was exhibited.
• No alterations at medium and low doses in the Peyers patches were observed; however, at high doses (HD) of P-MAPA this structure was prominent.
• The histopathological examinations of lymphonodes showed to be normal without any evidence of secondary follicles. Meanwhile, the analysis showed an increase in functionality, since the cortex and paracortex zones exhibited a dense cellular population, with predominance of the reticular cells and B lymphocytes. The cordons and medullar sinus were more dense in the P-MAPA treated animals that those lymphonodes from control and testimony animals.
• No morphological changes were found in thymus after treatment with P-MAPA, and the tissue revealed to be normal and with the same characteristic of those removed from control and testimony animals. The thymus weights were altered during the treatment.
• Spleen in low and medium dose exhibited normally in relation to control and testimony animals. However, the lymphoid cells (B and T) were in high level, around the penicillata artery and germinative centres. This was evidenced with the presence of numerous reticulo-endothelial cells systems (macrophils) and an extended cytoplasm by hepatic pigments, when compared with control and testimony animals. At HD a hyperfunction of the splenic phagocytic and macrophagic system was observed, associated to necrosis of lymphonod elements and the numerous presence of megakariocytes.
• These observations were proportional to the dose used of P-MAPA and were due to an activation of the immunological system.


|