

 Home |
Site Map |
Add to Favorites Link |
Send page to a Friend |
Contact us
Home |
Site Map |
Add to Favorites Link |
Send page to a Friend |
Contact us

|

 Home |
Site Map |
Add to Favorites Link |
Send page to a Friend |
Contact us
Home |
Site Map |
Add to Favorites Link |
Send page to a Friend |
Contact us
|
| About Us | Products | Research and Development | Publications and Press | Clinical Trials | Latest News | Contact us |
 |

05/04/2016
• Effects of P-MAPA on prostate cancer
• 20% of low, 0% of intermediary and 0% of high grade tumors
Overview
Prostate cancer is one the most common cancer worldwide. There is no "one size fits all" treatment for prostate cancer. Surgery, chemotherapy, radiotherapy and androgen deprivation therapy are the standard treatments. To discuss the patients qualify of life is also important, as people diagnosed with localized prostate cancer may stay alive for a long time during treatments.
As androgens have a pivotal role in the pathogenesis of prostate cancer, androgen-deprivation therapy (ADT) has been the backbone of locally advanced or metastatic prostate cancer treatment. However, after 15 to 24 months, most patients receiving ADT present a rise in prostate-specific antigen (PSA), indicating resistance to therapy.
As prostate tumors are also angiogenesis-dependent to grow and spread, angiogenesis inhibitors may help to reduce morbidity and mortality caused by such tumors. However, antiangiogenesis monotherapy has not yet proved to be beneficial to long-term survival (Ebos JML, 2011).
Therefore, there is an urgent need for new treatment strategies associating antiangiogenic agents with other treatments aiming the control of cancer. The association of angiogenesis inhibitors (e.g. TNP-470) with therapeutic agents that modulate steroid receptors (e.g. Flutamide) is a promising approach to reduce the disease recurrence and progression, as well as to improve the patients´ quality of life (Schweitzer & Drake, 2014).
Scientific rationale for the use of P-MAPA in prostate cancer
Previous studies with P-MAPA immunotherapy in association with antiandrogen therapy (Flutamide) showed that the interaction of steroid hormone receptors and their regulators, Siah2 and N-CoR, with the immune system receptors activated by P-MAPA resulted in remarkable therapeutic effects in an animal model for study of bladder cancer (Garcia et al., 2015).
Importantly, experimental evidences indicated the involvement of androgen receptor signals in the development of both bladder and prostate cancers. Other studies also pointed out a possible shared molecular carcinogenic process between these two cancers (Izumi et al., 2014).
Carcinoma in situ in the prostatic urethra is not uncommon in bladder cancer patients (Liedberg et al, 2004). Patients with bladder cancer have higher incidence of prostate cancer and vice versa, as these cancers may share a common carcinogenic process or patients are particularly susceptible to both cancers (Kinoshita et al., 2004; Izumi et al., 2014).
In this setting, previous results with P-MAPA - used alone and under conditions of antiandrogen therapy with Flutamide, a FDA-approved drug for treatment of prostate cancer - on bladder cancer showed an impressive therapeutic action and modulation of androgen receptors (Garcia et al, 2015).
Experimental data also have revealed the multiple ways by which P-MAPA can block tumor cell growth. In summary, P-MAPA activates pattern recognition receptors (PRRs) - specifically, Toll-Like Receptors 2 and 4 (TLR-2 and TLR-4) in vitro and in vivo - and induces a cascade of immune-mediated effects, responsible for the bladder cancer regression observed in animal models for study of bladder cancer.
The immune-mediated downstream cascade induced by P-MAPA after binding to TLRs leads to a restoration of p53 protein levels, induces tumor arrest and apoptosis, and notably blocks angiogenesis, by a strong inhibitory action of the vascular endothelial growth factor (VEGF)(Garcia et al, 2016).
In summary, P-MAPA acts on androgen receptors and also blocks angiogenesis in an animal model for study of bladder cancer. The results strengthen the possible use of P-MAPA immunotherapy in the treatment of prostate tumors, whose growth and spread are also androgen- and angiogenesis-dependent
Therefore, if the same results in bladder cancer are kept the experimental P-MAPA use may open new perspectives for the treatment of the disease in humans. The very low toxicity of P-MAPA may certainly help to preserve the patients´ quality of life.
Aims
The two aims of this study were:
1) to explore and compare the effects of P-MAPA as monotherapy and associated with an antiangiogenic therapy (TNP-470) in the treatment of chemically induced prostate lesions;
2) to verify if the experimental model for prostate cancer could reflect the impressive results of P-MAPA immunotherapy in the bladder cancer treatment.
Experimental Design
Thirty-five male F344/NTacUnib (Fischer 344) rats were used. The rats were housed individually with free access to ration and water. At 10 weeks of age the animals were randomly distributed into 7 groups. For induction of prostatic lesions, 20 animals ( 5 animals per group) received daily 100 mg/Kg dose of testosterone cypionate subcutaneously (s.c.) for 3 consecutive days.The other 15 animals were considered as Control group (Group 1, Group 2 and Group 3).
After the third day, the animals were anesthetized for inoculation of 15 mg/Kg dose of N-methyl-N-nitrosourea (MNU) in the ventral lobe of the prostate capsule. Two doses were applied, one by week.
One week after the last dose of MNU (Sigma Chemical Co., St Louis, MO, USA), the 20 animals received 5mg/kg dose of testosterone cypionate every day during 120 days.
After 120 days of prostate cancer (PCa) induction, all animals were submitted to ultrasound exams to check for presence of PCa and subdivided into seven groups (5 animals per group) as follow:
Control group (Group 1): the animals of this group received 5 ml/kg dose of 0.9% saline solution (s.c.), three times a week during 30 days;
Control+P-MAPA group (Group 2): the animals of this group received 5 mg/kg dose of P-MAPA (s.c.) (Farmabrasilis, Campinas, SP, Brazil), three times a week during 30 days;
Control+TNP470 group (Group 3): the animals of this group received 15mg/kg dose of TNP-470 (s.c.) (Sigma Chemical Co., St Louis, MO, USA), three times a week during 30 days;
Group MNU group (Prostate Cancer - Group 4): the animals of this group received the same treatment of the Group 1;
MNU+P-MAPA group (Group 5): the animals of this group received the same treatment of the Group 2;
MNU+TNP-470 group (Group 6): the animals of this group receive the same treatment of the Group 3;
MNU+P-MAPA+TNP- 470 group (Group 7): the animals of this group received treatment with P-MAPA and TNP-470, according to the same protocols applied in Groups 2, 3, 5 and 6.
After 150 days of treatment, samples of the ventral prostate lobe of all animals were collected and tested for macroscopic and histopathological analysis.
Histopathological Analysis
The ventral lobe (VL) of the control animals (Groups 1, 2 and 3) showed no macroscopic or microscopic changes (Figures 1a, 1b; Table -Groups 1,2,3). The VL was composed of acini with different sizes and pleated edge. The epithelium consisted of two cell types, one layer of basal cells and one of columnar secretory (luminal) cells.
The prostate stroma showed thin collagen fibers adjacent to the epithelium and interspersed with smooth cells (Figures 1a, 1b). All MNU-treated animals developed prostate cancer (PCa), 20% of them showed low, 60% intermediate and 20% high grade adenocarcinomas (Figure1 e,f,g,h, Table-Group 4).
Premalignant lesions such as high-grade prostatic intraepithelial neoplasia (HGPIN) and proliferative inflammatory atrophy (PIA) were observed in 80% of the animals (Figures 1c, 1d; Table -Group 4).
HGPIN was characterized by focal proliferation of the epithelium, cytologic atypia represented by large nuclei and prominent nucleoli. Basal cells showed no structural disorganization (Figures 1c, 1j). PIA was identified by atrophic acini with a dysplastic epithelium, with more than one layer of atypical cells, large nuclei and prominent nucleoli.
The epithelial alterations presented interstitial periacinar inflammatory infiltrate composed mainly of lymphocytes (Figures 1d, 1k). The basal cells did not present any structural alteration. The low-grade adenocarcinoma was predominantly composed of well-formed acini with discrete neoplasia similar to normal acini, but without basal cells (Figure 1e).
The nuclei of neoplastic acini were bulky and oval, with prominent nucleoli (Figure 1e). In the intermediate grade adenocarcinoma, there were no basal cells, neoplastic acini were sharp and began to merge (Figures 1f, 1l). High grade adenocarcinoma was characterized by rare acini, the neoplastic cells were arranged in cords or nests through stroma (Figures 1g, 1h).
Prostatic atrophy (PA) (Figure 1i) and nodular prostatic hyperplasia (NPH) were observed respectively in 100% and 60% of the MNU+TNP470 group. Premalignant lesions such as HGPIN and PIA were observed in 60% of the animals (Figures 1j, 1k; Table-Group 6).
The most frequent malignant lesions observed in the MNU+TNP470 group were intermediate grade adenocarcinoma (40%) (Figure 1l; Table-Group 6) and low (20%) and high (20%) grade prostatic adenocarcinoma (Table- Group 6).
A reduction of 80% in the occurrence of tumors was observed in the P-MAPA-treated group (Table-Group 5), which showed only low-grade adenocarcinoma (20%). In addition, P-MAPA reduced the occurrence of premalignant lesions (HGPIN and PIA), observed in 60% and 20% of the animals, respectively (Table-Group 5).
The most frequent lesion in P-MAPA group were Nodular prostatic hyperplasia (NPH) (80%) (Figure 1m; Table-Group 5); prostatic atrophy (80%) (Figure 1n; Table-Group 5) and inflammatory infiltration in the stroma (60%) (Figure 1o, Table - Group 5). In NPH, well-defined hyperplastic nodules characterized by acini with epithelial papillomatosis and rounded borders were observed (Figure 1m). The acini epithelium was composed of columnar mucin-secreting cells and basal cells (Figure 1m). The stroma of NPH was hipercellular and composed of thick collagen fibers (Figure 1m). In the prostatic atrophy, the acini showed no papillary projections and were composed mainly of cuboidal cells which presented a reduction in the nuclei/cytoplasm relation (Figure 1n). Basal cell layer was maintained (Figure 1n).
FIGURE 1
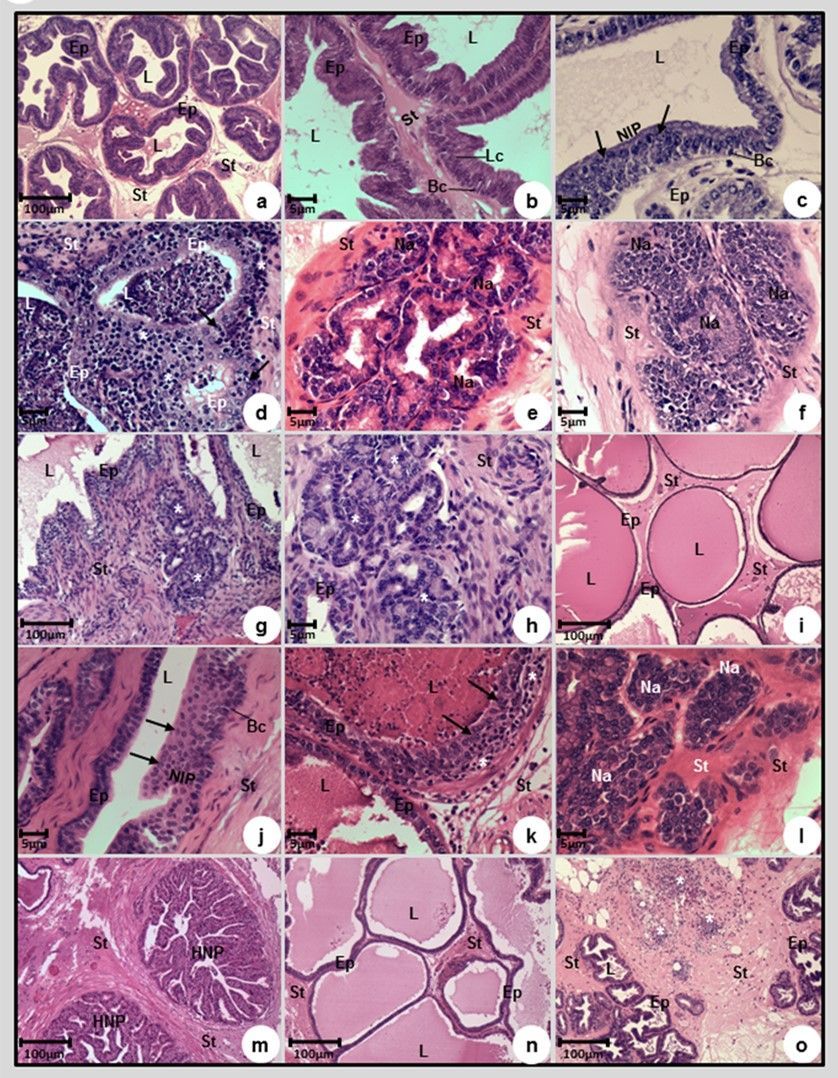
Figure 1: Photomicrographs of the prostatic ventral lobes of Control (a,b), MNU (c, d, e, f, g, h), MNU+TNP-470 (I, j, k, l) and MNU+P-MAPA (m, n, o) groups. (a, b) Acini with different sizes, pleated borders and epithelium composed by two cell types, one layer of basal cells (Bc) and one layer of columnar mucin-secreting cells (Lc). (c, j) Prostatic intraepithelial neoplasia (PIN) was characterized by focal proliferation of the epithelium, cytologic atypia, identified by large nuclei and prominent nucleoli (arrows); basal cells (Bc). (d, k) Proliferative inflammatory atrophy (PIA) was characterized by atrophic acini with a dysplastic epithelium, with more than one layer of atypical cell (with large nuclei and prominent nucleoli) (arrows). The epithelial alteration presented by interstitial periacinar inflammatory infiltrate mainly composed of lymphocytes (*). (e) Low-grade adenocarcinoma with well-formed acini, discrete neoplasia and cytologic atypia similar to normal acini, but without basal cells. (f, l) Intermediate degree adenocarcinoma was characterized by neoplastic sharp acini (Na) which began to merge, without basal cells. (g, h). High grade adenocarcinoma was characterized by rare acini, the neoplastic cells were arranged in cords or nests (*) through the stroma. (i, n) In prostatic atrophy, the acini showed no papillary projections and were composed mainly of cuboidal shape cells, which a reduction in the nuclei/cytoplasm relation, and the basal cell layer was maintained. (m) Nodular prostatic hyperplasia (NPH) was characterized by well-defined hyperplastic nodules, which were characterized by acini with epithelial papillomatosis and rounded borders. The acini epithelium was composed of columnar mucin-secreting cells and basal cells. (o) Inflammatory infiltrate (*) through the stroma. (a - o): epithelium (Ep), lumen (L) and stroma (St).
Table: Frequency of premalignant, malignant lesions and other histopathological findings in prostate
|
Groups |
Prostatic Lesions |
Prostate Cancer (Grade) |
|||||||
|
HGPIN |
PIA |
Micro acinus |
NPH |
PA |
Inflammatory Infiltrate |
Low |
Intermediate |
High |
|
|
Control Groups 1,2,3 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
Group 4 MNU |
80% |
80% |
- |
- |
- |
- |
20% |
60% |
20% |
|
Group 6 MNU + TNP470 |
60% |
60% |
- |
- |
100% |
- |
20% |
40% |
20% |
|
Group 5 MNU + P-MAPA |
60% |
20% |
60% |
80% |
80% |
60% |
20% |
0% |
0% |
|
Group 7 MNU + TNP470+ P-MAPA |
40% |
- |
100% |
40% |
60% |
60% |
40% |
40% |
0% |
(HGPIN): High grade prostatic intraepithelial neoplasia
(PIA): Proliferative Inflammatory Atrophy
(NPH): Nodular Prostatic Hyperplasia
(PA): Prostatic Atrophy
Benign Lesions: Microacinus; NPH; PA and Inflammatory Infiltrate.
Premalignant Lesions: HGPIN and PIA.
Malignant Lesions: Prostate Cancer: low grade, Intermediate grade and high-grade.
CONCLUSIONS
The histopathological data clearly demonstrated the noteworthy antitumor effect of P-MAPA monotherapy in prostate cancer (Figure 1, m,n,o and Table-Group 5).
Indeed, P-MAPA monotherapy eliminated the intermediary and high grade tumors in prostate. The P-MAPA-treated animals, after MNU-induced prostate lesions, presented only 20% of low grade tumors and 0% of both intermediary and high grade tumors.
In sharp contrast, the MNU-induced control group (Table-Group 4) presented 100% of tumors (20% of low, 60% of intermediary and 20% of high grade).
The results of P-MAPA monotherapy are coherent with those in bladder cancer treatment, which also showed an 80%-100% high grade tumor regression.Further work is ongoing to investigate if the mechanism of action of P-MAPA on prostate cancer is similar to those in bladder cancer.
In contrast, the TNP-470 monotherapy (Table-Group 6) and the association of P-MAPA and TNP-470 (Table- Group 7) presented a less significant effect than P-MAPA monotherapy against prostate tumors. The association of P-MAPA with TNP-470 (Group 6) was the best therapeutic approach only against premalignant lesions (HGPIN and PIA).
The experimental data also suggested that P-MAPA may interfere, directly or indirectly, in the hormonal pathways, as the compound reduced the proliferation of premalignant and malignant lesions. In addition, P-MAPA caused important changes in the tumor environment such as inflammatory infiltrate, prostatic atrophy and nodular prostatic hyperplasia.
Taking together, the experimental data suggest that prostate and bladder cancers could really have a common carcinogenic process, blocked by P-MAPA.
As bladder cancer patients can also have prostate cancer, the intravesical and systemic P-MAPA immunotherapy may provide a great chance of success to fight malignant lesions in the two sites at the same time.
Additional research is ongoing to elucidate the most appropriate ways to use antiandrogen therapies and/or chemotherapy in association with P-MAPA for the treatment of prostate cancer.


|